Providing Quality & Trust
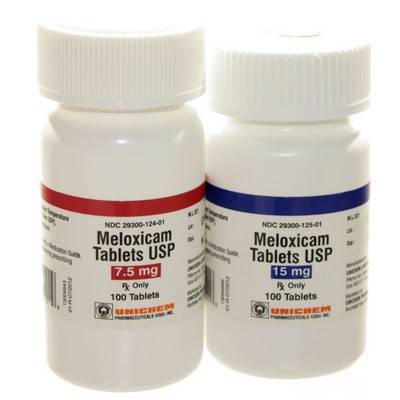
Meloxicam Tablet
Unichem
Starting at $0.17
$0.17 Each
Detailed Description
Meloxicam
(mel-ox-i-kam)
- Description: Non-Steroidal Anti-inflammatory Drug (NSAID)
- Other Names for this Medication: Metacam®, Mobic®
- Common Dosage Forms: Veterinary: 7.5 mg and 15 mg oral tablets.
Give this medicine with food; depending on size of the animal, the drug can be given either on top of food or directly into the mouth Ongoing (more than one dose) use of meloxicam in cats is controversial and it must be used cautiously, if at all.
Most animals tolerate this drug well; some will rarely develop ulcers or serious liver and kidney problems (especially cats).
Watch for: eating less than normal; vomiting; changes in bowel movements; changes in behavior or activity (less active than normal); weakness (stumbling, clumsiness); seizures (convulsions); aggression (threatening behavior and actions); yellowing of gums, skin, or whites of the eyes (jaundice); changes in drinking (frequency, amount consumed) or urination habits (frequency, color, or smell).
Store honey-flavored suspension well out of reach of animals and children. Periodic blood tests to check for liver and kidney side effects are required. Do not miss these importantfollow-up visits.
How is this medication useful?
Oral meloxicam suspension is used in animals to control pain and inflammation.
- The FDA has approved oral meloxicam suspension for use in dogs for the control of pain and inflammation associated with osteoarthritis.
- The FDA allows veterinarians to prescribe and use products containing this drug in different species or for other conditions in certain situations.
You and your veterinarian can discuss why this drug is the most appropriate choice.
Uses/Indications:
Meloxicam is used in the treatment of pain, inflammation, and osteoarthritis in dogs. Short-term use (eg, single-dose injectable, administered before surgery) is also FDA approved in the United States for cats for the control of postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy, and castration. Meloxicam is licensed in several countries for long-term use (at lower doses) in cats.
Oral meloxicam may be a cost-effective agent for use during painful procedures such as castration or dehorning in calves. However, meloxicam administration in calves prior to transportation did not affect movement or feeding or drinking behaviors and did not mitigate leukocyte function or inflammatory markers.
Metronomic cyclophosphamide and meloxicam successfully managed oral squamous cell carcinoma in a horse.
What are the side effects ofthis medication?
Meloxicam is tolerated well in most animals, but rarely, serious side effects (eg, stomach ulcers, liver or kidney problems) and sometimes death have been reported.
Side effects that may be serious or indicate a serious problem:
- Decrease in appetite (eating less than normal), vomiting, changes in bowel movements (such as diarrhea, or black, tarry or bloody stools).
- Changes in behavior or activity (less active than normal), incoordination, weakness (eg, stumbling, clumsiness), seizures (convulsions), or aggression (threatening behavior and actions).
- Yellowing of gums, skin, or whites of the eyes (jaundice).
- Changes in drinking (frequency, amount consumed) or urination habits (frequency, color, or smell).
- Changes in skin (eg, redness, scabs, scratching)
- Cats are more likely to develop serious kidney problems than dogs when the drug is used on an ongoing basis.
If you have any other questions about this medication, contact your veterinarian or pharmacist.
Contraindications/Precautions/Warnings:
Meloxicam is contraindicated in animals hypersensitive to it. The European and UK labels state that safe use has not been evaluated in dogs younger than 6 weeks of age; the US FDA label states safe use has not been established in dogs younger than 6 months old. The drug should not be used or should be used with extreme caution in dogs with active GI ulceration or bleeding or in dogs receiving glucocorticoids. To avoid accidental overdosing, dogs weighing less than 4.5 kg (10 lb) should have meloxicam doses carefully measured and applied on food only, never directly into the mouth.
Meloxicam, like all NSAIDs, should be used with caution in animals with impaired hepatic, cardiac, or renal function and animals with hemorrhagic disorders. Hypotension, high doses, hypovolemia, sodium depletion, and inhalant anesthesia all appear to increase risk for NSAID renal toxicity.
Concurrent use with other anti-inflammatory drugs may result in additional or increased adverse effects; therefore, a treatment-free period from all other anti-inflammatories should be observed for at least 24 hours before beginning treatment with meloxicam. The treatment-free period, however, should take into account the pharmacokinetic properties of the products used previously. Use meloxicam under strict veterinary monitoring in animals with a risk for GI ulcers, or if the animal previously displayed intolerance to other NSAIDs.
Meloxicam is contraindicated in cats with known hypersensitivity to meloxicam or other NSAIDs. The manufacturer warns that additional doses of meloxicam or other NSAIDs are contraindicated, as no safe dose for repeated NSAID administration has been established. Safe use in cats younger than 4 months of age has not been established. Preoperative use in cats undergoing major surgery, in which hypotensive episodes are possible, may result in a higher risk for renal damage.
The human label states that no dose adjustment is necessary in patients with mild to moderate hepatic or renal impairment.
The veterinary suspension comes with a dosing syringe that is marked in lb. If providing dosing directions in mL, make sure to supply an appropriately marked dosing syringe.
Adverse Effects:
Experience in Europe and Canada has demonstrated a relatively safe adverse effect profile for meloxicam at labeled doses in healthy dogs. GI distress is the most commonly reported adverse effect, and in US field trials, vomiting, soft stools, diarrhea, and inappetence were the most common adverse effects reported. Renal toxicity appears to be quite low in animals with normal renal blood flow. Adverse effects reported postapproval have included GI effects (eg, vomiting, anorexia, diarrhea, melena, ulceration), elevated liver enzymes/hepatotoxicity, pruritus, azotemia, elevated creatinine, and renal failure. When NSAIDs are used chronically in dogs, gastroprotectant drugs (eg, proton pump inhibitors [PPIs], H2 blockers, misoprostol) have been used in an attempt to prevent or limit GI adverse effects, but the benefits of these drugs have not been proven. Like other COX-2 NSAIDs, meloxicam may have effects on platelet function, although no effect was demonstrated in healthy dogs in a small study. A case report of a dog developing vasculitis with ulcers, vesicles, and erosions has been published. In cats, single doses of meloxicam appear relatively safe. In field trials, some cats developed elevated BUN, posttreatment anemia, and residual pain at the injection site (rare). In other studies, meloxicam has caused GI effects (eg, vomiting, diarrhea, inappetence), behavior changes, and lethargy. Repeated use of meloxicam in cats is controversial, as repeated doses have been associated with renal failure and death. The FDA warns against repeated doses in cats. However, low-dose chronic use is licensed in some countries, and the International Society of Feline Medicine (ISFM) and American Association of Feline Practitioners (AAFP) guidelines for long-term NSAID use in cats suggest the benefits of treatment often outweigh the risks.
Acute dosing studies in dogs have not demonstrated any hepatic toxicity. Use over 2 months in piglets demonstrated no adverse effects on trabecular bone or growth plates.
Meloxicam is relatively safe in other species (eg, horses, cattle, swine, rabbits, small ruminants, camelids) when used at recommended doses.
Drug Interactions:
In humans, meloxicam is a CYP2C9 substrate. The following drug interactions have either been reported or are theoretical in humans or animals receiving meloxicam and may be of significance in veterinary patients. Unless otherwise noted, use together is not necessarily contraindicated, but weigh the potential risks and perform additional monitoring when appropriate.
- ACE INHIBITORS (eg, benazepril, enalapril): NSAIDs can reduce effects on blood pressure.
- AMIODARONE: May increase meloxicam levels
- AMLODIPINE: Some NSAIDs can reduce effects on blood pressure.
- ANESTHETICS, INHALANT: May increase risk for renal hypotension and NSAID renal toxicity
- ANGIOTENSIN-II RECEPTOR BLOCKERS (ARB; eg, telmisartan): Some NSAIDs can reduce effects on blood pressure.
- ANTICOAGULANTS (eg, heparin, rivaroxaban, warfarin): Increased chance for bleeding
- BISPHOSPHONATES (eg, alendronate): May increase risk for GI ulceration
- CLOPIDOGREL: Increased chance for bleeding
- CORTICOSTEROIDS (eg, prednisone): May increase risk for GI toxicity (eg, ulceration, bleeding, vomiting, diarrhea); avoid use with NSAIDs
- CYCLOSPORINE: Concurrent use may result in increased risk for cyclosporine nephrotoxicity.
- DIGOXIN: NSAIDs may increase serum levels.
- DIURETICS (eg, furosemide, hydrochlorothiazide): Concurrent use may result in reduced diuretic efficacy and possible nephrotoxicity.
- FLUCONAZOLE: Administration has increased plasma levels of celecoxib in humans and could also affect meloxicam levels in dogs.
- FLUOROQUINOLONES (eg, enrofloxacin, marbofloxacin): Meloxicam may increase fluoroquinolone levels, increasing risk for CNS toxicity.
- LEFLUNOMIDE: May increase meloxicam exposure; risk for leflunomide hepatotoxicity may be increased
- METHOTREXATE: Concomitant NSAID use with methotrexate may increase risk for myelosuppression, nephropathy, hepatotoxicity, and GI toxicity; use together with extreme caution.
- NEPHROTOXIC DRUGS (eg, aminoglycosides, amphotericin B, furosemide): May enhance risk for nephrotoxicity
- NSAIDs, OTHER: May increase risk for GI toxicity (eg, ulceration, bleeding, vomiting, diarrhea)
- PENTOXIFYLLINE: May increase risk for bleeding
- SALICYLATES (eg, aspirin, bismuth subsalicylate): Concurrent use may increase risk for GI toxicity and bleeding.
- SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs; eg, fluoxetine): Concurrent use may result in an increased risk for bleeding.
- SULFONAMIDES (eg, sulfamethoxazole): May inhibit meloxicam metabolism, increasing meloxicam exposure and risk for toxicity
- TRICYCLIC ANTIDEPRESSANTS: Concurrent use may result in increased risk for bleeding.
Storage/Stability:
Unless otherwise labeled, store the injection and oral liquid at controlled room temperature (68°F-77°F [20°C-25°C]), with excursions permitted between 59°F and 86°F (15°C and 30°C) for the oral suspension. The oral suspension labeled for use in the United Kingdom has a 6-month shelf life after the immediate package is first opened.

Powered by nopCommerce
This site is running in live payment mode. Real payments will be processed.