Providing Quality & Trust
Incurin Estriol Tablets
Merck
Starting at $34.98
$34.98 Each
Detailed Description
INCURIN® TABLETS (estriol)
A treatment for female canine urinary incontinence that contains estriol, a naturally occurring, short-acting estrogen found in female dogs with the convenience of once-a-day dosing.
Incurin is indicated for the control of estrogen-responsive urinary incontinence in ovariohysterectomizedfemale dogs.
Features and Benefits:
- Proven effective in reducing or eliminating urinary incontinence
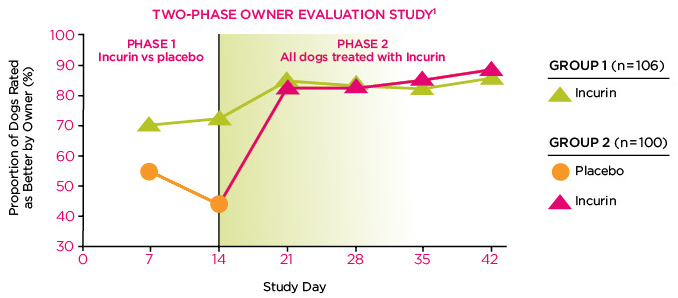
- 93% of dogs treated were improved or continent by 6 weeks
- Convenient, once-a-day dosing
- One simple starting dose for all female dogs and just 1 tablet size to stock
- Titrated to the lowest effective dose
Efficacy:Incurin (estriol) Tablets have been proven to reduce or eliminate urinary incontinence in spayed female dogs. Incurin helps restore urinary continence—The majority of owners participating in a study of over 200 subjects felt their dogs improved after being treated with Incurin.
|
 |
Administration and Dosage:
 |
|
Fair Balance:
Incurin is indicated for the control of estrogen-responsive urinary incontinence in ovariohysterectomized dogs. The most common side effects associated with Incurin treatment under field conditions included loss of appetite, vomiting, excessive water drinking, and swollen vulva. The safety and effectiveness of Incurin Tablets have not been evaluated in dogs less than 1 year of age, intact female dogs, male dogs, dogs used for breeding, or lactating dogs. Incurin is contraindicated in dogs showing polyuria secondary to polydipsia, or in pregnant dogs. Please see the product label for full prescribing information.
For additional information, please see the product label.
Supplied:
12x blister (30 x 1 mg)
Active Ingredients:
- Estriol
Side Effects:
The most common side effects associated with Incurin treatment under field conditions included loss of appetite, vomiting, excessive water drinking, and swollen vulva.
The safety and effectiveness of Incurin Tablets have not been evaluated in dogs less than 1 year of age, intact female dogs, male dogs, dogs used for breeding, or lactating dogs.
Safety Information:
- Well tolerated in a long-term field study (42-month) involving 324 client-owned ovariohysterectomized female dogs.
- Safely used worldwide for more than 16 years
- Short receptor binding time limits estrogen-related side effects.
For further information, including complete directions and warnings, please see the full prescribing information.

Powered by nopCommerce
This site is running in live payment mode. Real payments will be processed.